Your cart is currently empty!
Evaluating the impact of universal Lynch syndrome screening in a publicly funded healthcare system
All your purchased models will be available under your account under “Dashboards”
Disease Area (Primary)
Lynch syndrome
First Developed
07/23/2020
Last Developed
07/23/2020
Software Used
R (e.g., heemod, BCEA, dampack, hesim)
Model Sponsor
Unknown
Intervention
universal_screening
Model Validation Score
20 %
Coming Soon In Phase II: You will be able to pay a fee to download the CADTH Tool for your model which includes subaggregated scores.
Results
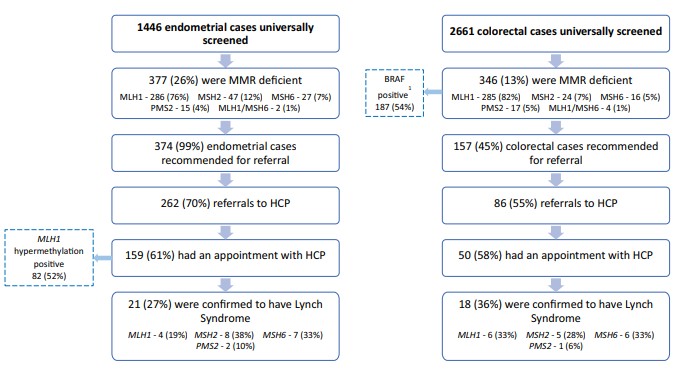
The mutation detection rate of the universal screening group was higher than the traditionally referred group (45/228 (19.7%) vs 50/390 (12.5%), P = .05), though each were able to identify unique patients. An analysis of testing criteria met by each patient showed that half of referred patients from the universal screening group could not meet any traditional testing criteria.
Conclusion
The implementation of universal screening in a publicly funded system will increase efficiency in detecting patients with LS.
Source File(s)