Your cart is currently empty!
Clinical and Economic Impact of Molecular Testing for BRAF Fusion in Pediatric Low-Grade Glioma
All your purchased models will be available under your account under “Dashboards”
Disease Area (Primary)
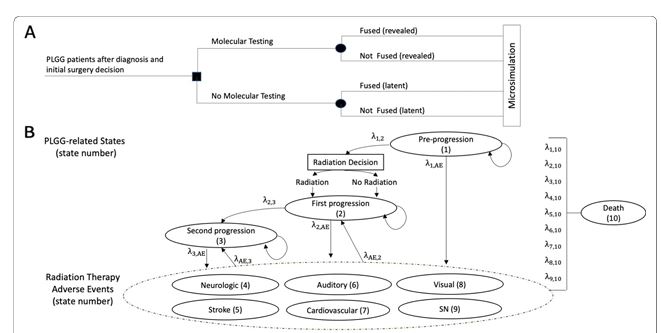
Pediatric low-grade glioma (PLGG)
First Developed
06/07/2020
Last Developed
10/27/2021
Software Used
R (e.g., heemod, BCEA, dampack, hesim)
Model Sponsor
Healthcare organization
Intervention
braf_molecular_testing
Model Validation Score
– %
Coming Soon In Phase II: You will be able to pay a fee to download the CADTH Tool for your model which includes subaggregated scores.
Results
The life expectancy after diagnosis for individuals who did not receive molecular testing was 39.01 (95% Confidence Intervals (CI): 32.94;44.38) years and 40.08 (95% CI: 33.19;45.76) years for those who received testing. Our findings indicate that patients who received molecular testing at diagnosis experienced a 0.38 (95% CI: 0.08;0.77) gain in QALYs and $1384 (95% CI: $-3486; $1204) reduction in costs over their lifetime. Cost and QALY benefits were driven primarily by the avoidance of long-term adverse events (stroke, secondary neoplasms) associated with unnecessary use of radiation.
Conclusion
What are the key conclusions or current applications of this model?
Source File(s)